The physical properties of the water
It is very important to know that the water has a higher melting point , boiling point , and heat of vaporization than most common liquid . This fact indicates that there are strong forces of attraction between the adjacent water molecules .
The Water exists in three states
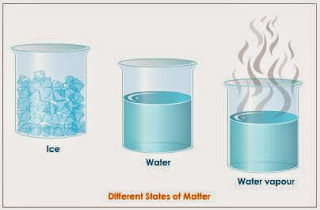 |
| The states of the water |
You should know that the states of the water are the solid state ( ice ) , the liquid state ( the water ) , and the gaseous state ( the water vapour ) at the normal temperature .
The water is a good polar solvent
 |
| The water dissolves the most ionic compounds such as the table salt . |
You notice that the water is a good polar solvent . So , It has a great ability to dissolve the most ionic compounds such as the table salt ( sodium chloride ) .
The water can also dissolve some covalent compounds such as the sugar that can form the hydrogen bonds with it . Some covalent compounds such as oil can not dissolve in the water as they can not form the hydrogen bonds with the water .
 |
| The water is a good polar solvent . |
The Pure water boils at 100 degrees Celsius and freezes at 0 degrees Celsius
You should know that the high boiling point and the low freezing point of the water is due to the presence of the hydrogen bonds between its molecules .
The water density decreases on freezing
 |
| The ice floats on the water surface which helps the aquatic creatures to be alive . |
It is very important to know that the density of the water in the solid state ( the ice ) is lower than its density in the liquid state as when the temperature of the water deceases than 4 degrees Celsius , The water molecules are collected together by the hydrogen bonds to form the ice crystals which have the hexagonal shape , the large volume and the large number of spaces between them .
You should know that the ice crystals float on the water surface and this helps in the preservation of the life of the aquatic creatures in it .
The water has high latent heat
It is very important to know that the water has high latent heat . So , it resists the change from a state to another . So , the water is used to extinguish the fires , as it consumes a large amount of the heat of the combustion media during its vaporization process .
The water has high specific heat
You notice that the temperature of the human body does not change by changing the atmospheric temperature . The high specific heat of the water makes it absorb or lose the large amount of the heat without changing its temperature .
The chemical properties of the water
The weakness of the water ionization
 |
| The water ionizes to give positive hydrogen ions and negative hydroxide ions . |
You should know that the ionization is the process of converting the molecules of some covalent compounds into ions , And The pure water is considered from the weakly ionized that gives positive hydrogen ions and negative hydroxide ions .
The water has a neutral effect
 |
| The water has a neutral effect on the litmus paper . |

No comments:
Post a Comment