The electronic distribution
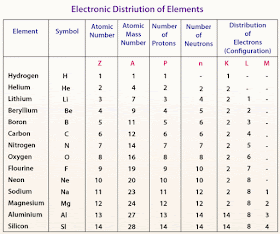 |
| Electronic distribution of elements . |
It is very important to know that each energy level can take a definite number of electrons , the number of electrons which saturates the first four energy energy levels can be calculated .
You should know that you can not calculate the number of electrons of the energy levels higher than four because the atom is not stable .
 |
| Electronic configuration . |
It is very important to know that the outermost energy level of any atom can not take more than 8 electrons except ( K ) level which is saturated with 2 electrons only .
 |
| Energy levels |
You should know that the electrons are distributed in the first level ( K ) which is the least energy , then the second and so on .
 |
| Electronic distribution of elements |
What is the relation between the electronic configuration and the chemical activity ?
You should know that the chemical activity is related to the number of electrons in the outer level , where if the number of the electrons in the outermost energy level of an atom is less than 8 electrons , the atom is unstable ( active ) .
 |
| The atom |
You should know that when the atom is unstable , it reacts chemically with another atom or more than one atom to produce a molecule in a stable state .
It is very important to know that if the number of electrons in the outermost energy level of an atom equals 8 electrons like those of inert ( noble ) gases , the atom will be inactive , so it does not react chemically with other elements .
You should know that the electrons of the outermost energy level are responsible for the chemical reactions .
 |
| Hydrogen and Helium |
It is very important to know that Helium is the only inert gas that has 2 electrons in the outermost energy level ( K ) level .
Active gases
 |
| Reactive elements and inert elements . |
You should know that atoms of active elements take part in the chemical reactions to produce stable molecules , Their molecules are formed of two atoms , and the outer level contains less than 8 electrons .
Inactive gases
You should know that the noble gases do not enter a chemical reaction through ordinary conditions as the outermost energy levels are completely filled with the electrons ( 8 electrons ) except ( He ) , Their molecules are formed of one atom .
 |
| Noble gases are in group 18 in the periodic table . |
No comments:
Post a Comment